18+ Calculating Reaction Free Energy Under Nonstandard Conditions
000003500 000003510 reaction vessel with 96 atmospheres of 000006019 000006029 methane 0699 atmospheres of oxygen 828 000011870 000011880 atmospheres of carbon. These plots show the free energy.
Free Energy Under Nonstandard Conditions Introductory Chemistry 1st Canadian Edition
182e calculating reaction free energy under nonstandard conditions.
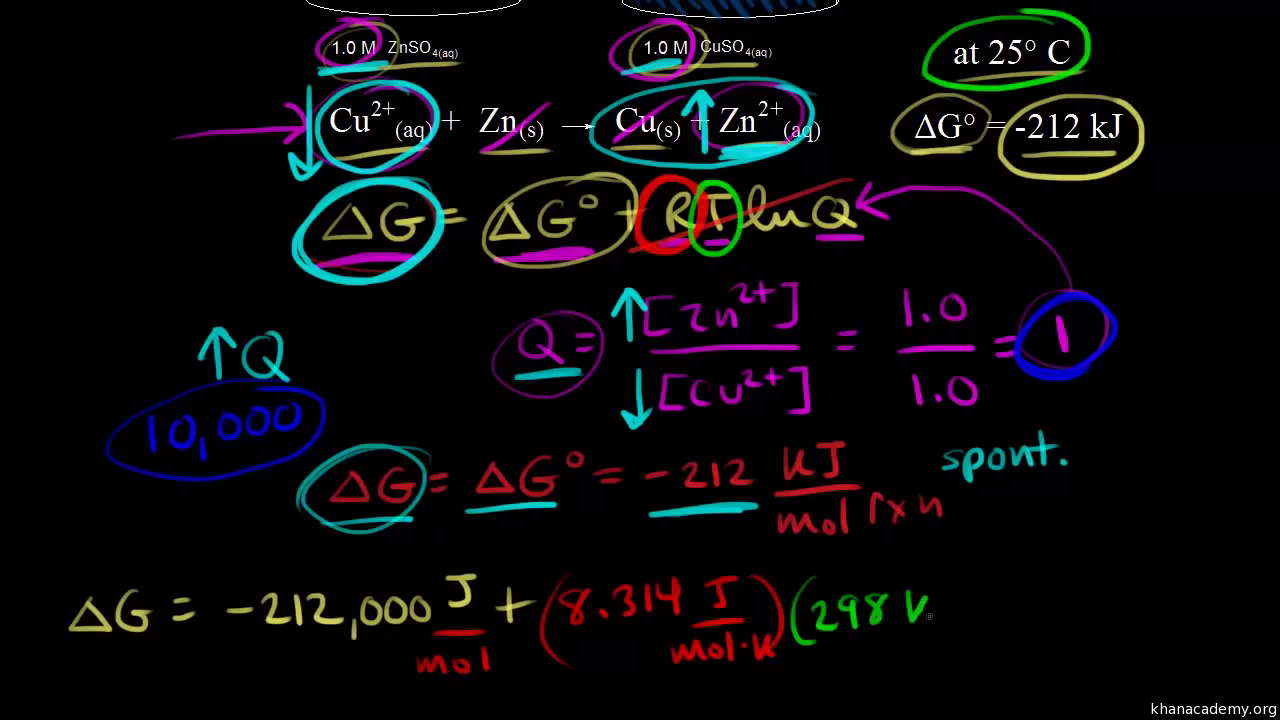
. Under standard conditions the reactant and product solution concentrations are 1 M or the pressure of gases is 1 bar and Q is equal to 1. A ΔGrnxΔGrnxRTlnQ B ΔGrnxΔGrnxRTlnQ C ΔGrnxΔGrnxRTlnQ D. The standard free energy change for a chemical reaction occurring under the standard condition can be calculated in one of three ways.
The free energy at nonstandard conditions can be determined using δg δg rt ln q. 182e Calculating reaction free energy under nonstandard conditions - YouTube AboutPressCopyrightContact usCreatorsAdvertiseDevelopersTermsPrivacyPolicy SafetyHow YouTube. To find the total free energy destroyed when a reactant is consumed or created when a reactant is produced we must multiply the free energy per mole which is given by by the number of.
How do you calculate the change in free energy for a reaction under nonstandard conditions. Single- and Double-Displacement Reactions. Under standard conditions the reactant and product solution concentrations are 1 M or the pressure of gases is 1 bar and Q is equal to 1.
Taking the natural logarithm simplifies the. Taking the natural logarithm simplifies the. Composition Decomposition and Combustion Reactions.
Now the reaction quotient and the Gibbs free energy for standard-state conditions are combined into. Types of Chemical Reactions. About Press Copyright Contact us Creators Advertise Press Copyright Contact us Creators Advertise.
Q K Reaction tends to form more reactants. Under standard conditions the reactant and product solution concentrations are 1 M or the pressure of gases is 1 bar and Q is equal to 1. Q K Reaction is already at equilibrium.
Taking the natural logarithm simplifies the.

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

Calculating Reaction Free Energy Under Nonstandard Conditions副本 16 12 4 上午 1 37 Aleks 第 1 共 4 Course Hero
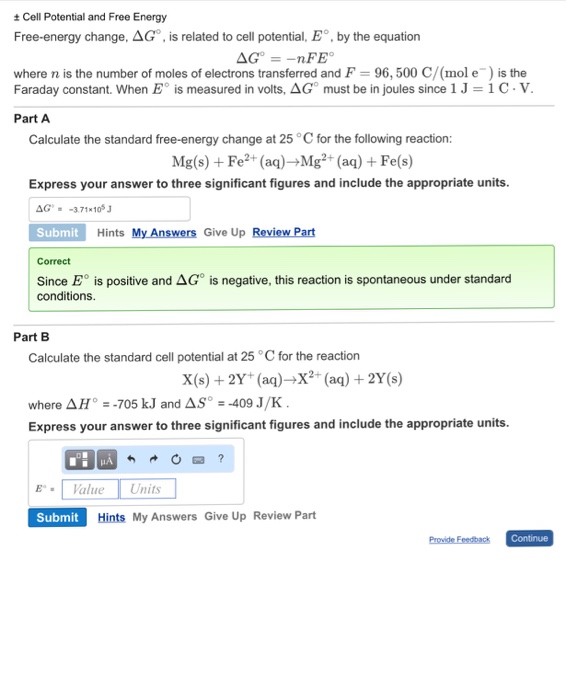
Solved Plusminus Cell Potential And Free Energy Free Energy Chegg Com

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

Solved O Entropy And Free Energy Calculating Reaction Free Chegg Com
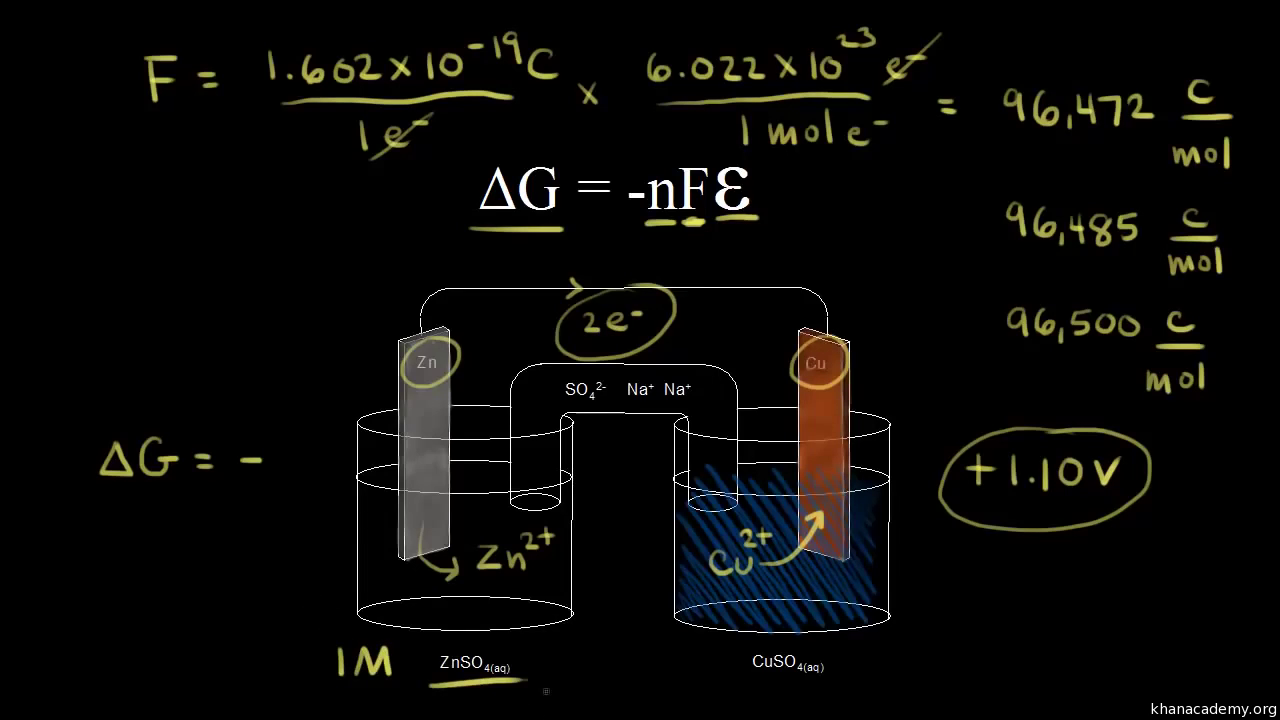
Free Energy And Cell Potential Video Khan Academy

Gibbs Free Energy Nonstandard Derivation Youtube
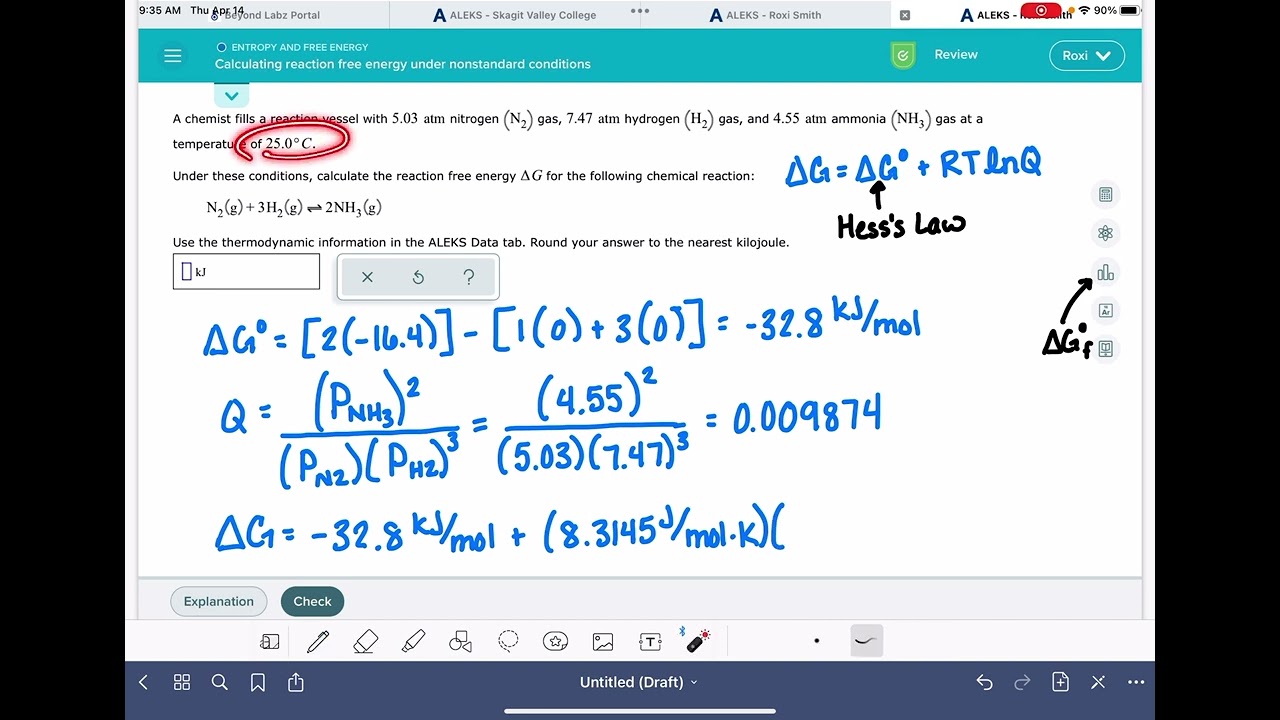
Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

18 2e Calculating Reaction Free Energy Under Nonstandard Conditions Youtube
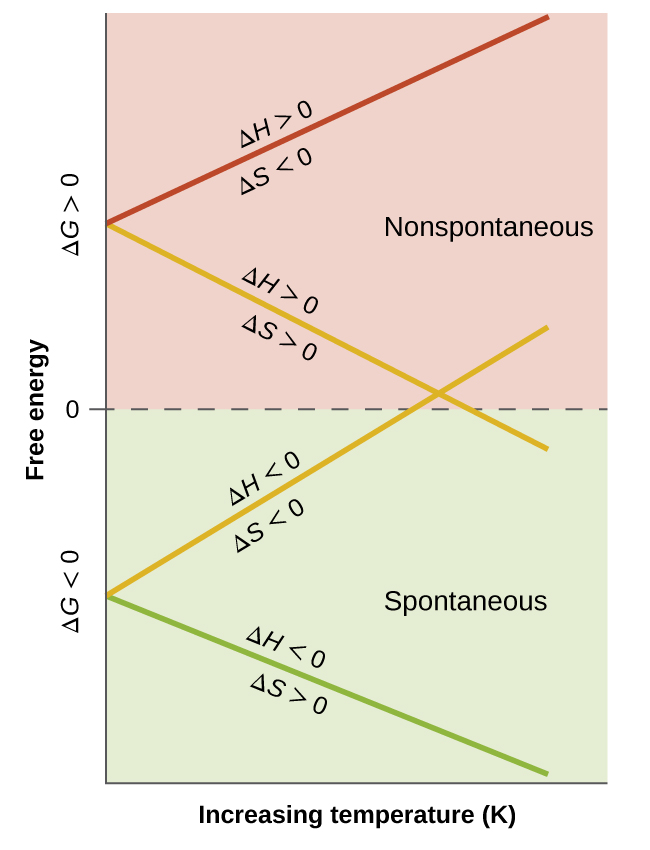
16 4 Free Energy 2018 Chemistry 112 Chapters 12 17 Of Openstax General Chemistry

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com

19 6 Free Energy Under Non Standard Conditions Youtube

Calculate Nonstandard Free Energy Youtube

How Does This Equation From Gibbs Free Energy Differ From G Rtlnkeq R Mcat
Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K Youtube
How Much Energy Is Released In Atp Hydrolysis

Galvanic Cells And Changes In Free Energy Video Khan Academy